Dr. Smith is Director, Cancer Screening, American Cancer Society, Atlanta, GA. Dr. Saslow is Director, Breast and Gynecologic Cancer, American Cancer Society, Atlanta, GA. Ms. Andrews Sawyer is Cancer Control Researcher, American Cancer Society, Atlanta, GA. Dr. Burke is Chair and Professor of Medical History and Ethics, University of Washington, Seattle, WA. Dr. Costanza is Professor of Medicine, University of Massachusetts Medical School, Worcester, MA. Dr. Evans is Director of Breast Imaging, University of Texas Southwestern Center for Breast Care, Dallas, TX. Dr. Foster is Wadley R. Glenn Professor of Surgery, Retired, Emory University School of Medicine, Atlanta, GA. Dr. Hendrick is Research Professor and Director, Breast Imaging Research, Department of Radiology, Lynn Sage Comprehensive Breast Center and the Northwestern Memorial Hospital, Chicago, IL. Dr. Eyre is Chief Medical Officer and Executive Vice President, Research and Cancer Control, American Cancer Society, Atlanta, GA, and Editor in Chief of CA. Dr. Sener is Vice Chairman, Department of Surgery, Evanston Northwestern Healthcare, Evanston, IL. This article is available online at: http://CAonline.AmCancerSoc.org
American Cancer
Society Guidelines
for Breast Cancer Screening:
Update 2003
Robert A. Smith, PhD; Debbie Saslow, PhD; Kimberly
Andrews Sawyer;Wylie Burke,MD, PhD
(for the High-Risk Work Group); Mary E. Costanza, MD
(for the Screening Older Women Work
Group);W. Phil Evans III, MD (for the Mammography Work
Group); Roger S. Foster, Jr.,MD
(for the Physical Examination Work Group); Edward
Hendrick, PhD (for the New Technologies
Work Group); Harmon J. Eyre,MD; Steven Sener, MD (for
the Breast Cancer Advisory Group)
ABSTRACT In 2003, the American Cancer Society updated
its guidelines for early detection
of breast cancer based on recommendations from a formal
review of evidence and a recent
workshop. The new screening recommendations address
screening mammography, physical
examination, screening older women and women with
comorbid conditions, screening
women at high risk, and new screening technologies. (CA Cancer J Clin 2003;54:141-169.)
© American Cancer Society, 2003.
INTRODUCTION
The underlying premise for breast cancer screening is that it allows for
the
detection of breast cancers before they become palpable. Breast cancer is
a
progressive disease, and small tumors are more likely to be early stage
disease, have a
better prognosis, and are more successfully treated.1 In this document, we use the
term screening to refer to the testing of asymptomatic individuals for the detection of
occult disease. Early detection means the application of a technique or
strategy that
results in earlier diagnosis of nonpalpable, as well as palpable, breast
cancers than
otherwise would have occurred.
The efficacy of breast cancer screening has been demonstrated in
randomized
controlled trials (RCTs) and observational studies; thus, most
organizations that issue
recommendations endorse regular mammography as an important part of
preventive
care.However, while it is true that screen-detected breast cancers are
associated with
reduced morbidity and mortality, the majority of women who participate in
screening will not develop breast cancer in their lifetime. Screening
also will not
benefit all women who are diagnosed with breast cancer, and it leads to
harms in
women who undergo biopsy for abnormalities that are not breast cancer, as
well as
those who are overtreated for ductal carcinoma in situ (DCIS) that might
have been
nonprogressive. Thus, in addition to benefits, limitations of screening
and harms
associated with screening are addressed in this guideline update.
Author disclosures: Dr.
Runowicz receives speaking fees and research support from Cytyc Corporation
(First
Cyte Ductal Lavage). Dr. Rubinstein is on the speakerís bureau for
Myriad Genetic Laboratories, Inc. Dr. DíOrsi is
a medical consultant to GE Medical Systems and R2 Technology, Inc. Dr.
Feig is on the medical advisory board of
R2 Technology, Inc., a company that sells a computer-aided detection
device for mammography; he does not receive
any financial remuneration or grant support from the company. Dr. Giger
is a shareholder in R2 Technology, Inc.;
she also has received unrestricted research support from the company in
the past.
In 1997, the American Cancer Society
(ACS) updated its guidelines for breast cancer
screening.2 The
most notable change in the
1997 guideline update was the recommendation
that women should begin annual
screening at age 40; the previous guideline had
recommended mammography every one to
two years for women beginning at age 40, and
annual mammography for women beginning at
age 50.3 The
1997 update also noted that there
was no chronological age at which screening
should stop, emphasizing that as long as a
woman was in good health she likely
would benefit from breast cancer screening.
Recommendations for clinical breast examination
(CBE) were modified by adding the
advice that women 40 and older schedule
annual CBE close to the time of, and before,
their annual mammograms.2
Guideline Development
In 2002, the ACS convened an expert panel
to review the existing early detection guidelines
based on evidence that has accumulated since
the last revision. The panel was divided into
work groups to review recent evidence and
develop recommendations regarding: (1)
mammography; (2) physical examination; (3)
screening of older women and women with
comorbid conditions; (4) screening high-risk
women; and (5) screening with new technologies.
During the current guideline review,
literature related to breast cancer screening
published between January 1997 and
September 2002, including new screening tests,
was identified using MEDLINE (National
Library of Medicine), bibliographies of
identified articles, personal files of panel
members, and unpublished manuscripts. Expert
panel members reviewed articles using specified
criteria and discussed them during a series of
conference calls. Each work group developed
recommendations, rationale, and evidence
summaries, and reviewed the summaries developed
by the other work groups prior to a
September 2002 workshop. When evidence
was insufficient or lacking, the final
recommendations incorporated the expert
opinions of the panel members. During the
conference calls and workshop, consensus was
reached on the key issues within the guideline
recommendations. Following the workshop,
ACS Breast Cancer Advisory Group members
deliberated over the guideline modifications.
Each work group member and workshop
attendee was given the opportunity to review
the draft of this manuscript. Numerous
professional, advocacy, and governmental
organizations also were invited to review the
draft guidelines.
RECOMMENDATIONS, RATIONALE, AND EVIDENCE
Summary of Guidelines
A summary of the update of the ACS
guidelines for early breast cancer detection is
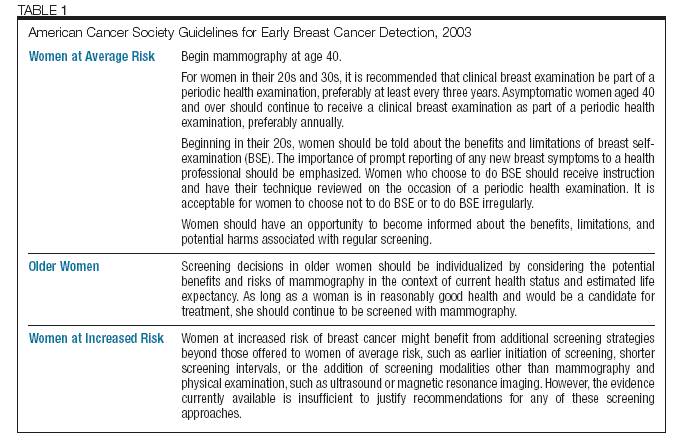
shown in Table 1.
SCREENING WITH MAMMOGRAPHY
Recommendation
Women at average risk should begin annual
mammography at age 40.Women should have
an opportunity to become informed about
the benefits, limitations, and potential harms
associated with regular screening.
Rationale and Evidence
Since 1997, there have been updates in
the evidence from RCTs of breast cancer
screenings. Several other reports have
challenged the value of screening for breast
cancer with mammography,4-7 leading to a surge
of new literature reexamining the underlying
updated clinical trial results from individual studies
and meta-analyses continue to show a significant
mortality reduction from mammography screening,
and this finding is further supported by evidence
from organized screening programs.
